创建自己的小题库
搜索

元素的推断题库
题数
392
考试分类
高中化学>元素的推断
售价
¥20
 手机预览
手机预览
收藏
分享
去刷题
简介
高中化学-元素的推断
...更多



0道

0道

0道



章节目录
题目预览(可预览10题)
【简答题】

[1/392]有A、B、C、D、E、F六种短周期元素,他们的原子序数依次增大,B与A能以原子个数1:1、1:2、1:3或1:4等比值组成多种常见化合物;C的最髙正价...
参考答案:
| (1)第三周期VIA族 (2)  (3)NH3和H2O都为极性分子,相似相溶;NH3分子和H2O分子间易形成氢键 (4)c(NH4+)﹥c(Cl-)﹥c(OH-)﹥c(H+) (5)CH3CH2OH-12e-+16OH-=2CO32-+11H2O (6)CH3CH2OH-12e-+ 3H2O =2CO2+12H+ (7)离子晶体;Na2S+2H2O  S↓+2NaOH+H2↑ S↓+2NaOH+H2↑ |
参考解析:
无
【简答题】

[2/392]短周期中的三种元素X、Y、Z,原子序数依次减小,原子核外电子层数之和是5,X元素原子的最外层电子数是Y和Z两元素原子的最外层电子数的总和;Y元素原子的...
参考答案:
| (1)N;C;H (2)强于;2HNO3+Na2CO3==CO2↑+H2O+2NaNO3 (3)  (4)NH3+H2O+CO2==NH4HCO3 |
参考解析:
无
【简答题】

[3/392]有A、B、C、D、E五种短周期元素,原子半径依次减小。E元素与其它元素不在同一周期。C、D在周期表中处于相邻位置,它们的单质在通常状况下均为无色气体。...
参考答案:
| (1)硫化钠 (2)H3O+ (3)SO2或H2O2 (4)2H2SO4(浓)+Cu 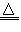 CuSO4+SO2↑+2H2O CuSO4+SO2↑+2H2O (5)2Na2O2 + 2H2O = 4NaOH + O2↑ 或 3NO2 + H2O = 2HNO3 + NO |
参考解析:
无
【简答题】

[4/392]A、B、C、D、E、F、G七种元素的原子序数依次增大且不超过20。在周期表中A的原子半径最小;D是地壳中含量最大的元素,B、D的原子序数之比为 3:4...
参考答案:
(I)分子晶体; (2)O=C=O; Ca2+ (3)②④ (4)Si +2OH- + H2O==SiO32- +2H2↑ (5)0.25;2.8 |
参考解析:
无
【简答题】

[5/392]有X、Y、Z、W四种短周期元素,原子序数依次增大,其核电荷数总和为38。Y元素原子最外层电子数占核外总电子数的3/4;W元素原子最外层电子比同周期Z元...
参考答案:
| (1)H;O;Mg;Cl (2)Mg(OH)2+2HClO4==Mg(ClO4)2+2H2O (3)有气泡、变红;Mg+2H2O  Mg(OH)2+H2↑ Mg(OH)2+H2↑ |
参考解析:
无
【简答题】

[6/392]a、b、c、d、e是短周期元素,周期表中a与b、b与c相邻;a与e的最外层电子数之比为2∶3,b的最外层电子数比e的最外层电子数少1个;常见化合物d2...
参考答案:
| (1)S (2)共价键和离子键;离子晶体;电子式“略” (3)CO32-+H2O  HCO3-+OH-或C2O42-+H2O HCO3-+OH-或C2O42-+H2O HC2O42-+OH- HC2O42-+OH-(4)0.3 mol Na2O2,0.1 mol Na2CO3 |
参考解析:
无
【简答题】

[7/392]已知A、B、C、D两种短周期元素,分别属于不同的主族,其原子序数依次增大,其中A与D、B与C的原子的最外层电子数之和均为9,A原子的最外层与次外层电子...
参考答案:
| (1)Be;H2S (2)Fe+S  FeS;2Fe+3Cl2 FeS;2Fe+3Cl2 2FeCl3(或H2S+Cl2==S↓+2HCl) 2FeCl3(或H2S+Cl2==S↓+2HCl)(3)2 (4)Al3++3AlO2-+6H2O===4Al(OH)3 |
参考解析:
无
【简答题】

[8/392]A、B、C均为短周期元素,可形成A2C和BC2两种化合物。A、B、C的原子序数依次递增,A原子的K层的电子数只有一个,B原子2p能级有一个空轨道,而C...
参考答案:
| (1)H;C;S (2)  (3)极性;非极性;sp |
参考解析:
无
【简答题】

[9/392]已知X、Y和Z三种元素的原子序数之和等于42。X元素原子的4p轨道上有3个未成对电子,Y元素原子的最外层2p轨道上有2个未成对电子。X跟Y可形成化合物...
参考答案:
| (1)1s22s22p63s23p63d104s24p3;As (2) 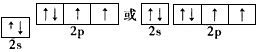 ;氧 ;氧(3)三角锥形 (4)As2O3+6Zn+6H2SO4==2AsH3↑+6ZnSO4+3H2O (5)稳定性:NH3> PH3> AsH3,因为键长越短,键能越大,化合物越稳定沸点:NH3> AsH3> PH3, NH3可以形成分子间氢键,沸点最高,AsH3相对分子质量比PH3大,分子间作用力大,因而AsH3的沸点比PH3高 |
参考解析:
无
【简答题】

[10/392]已知W、D、E、X、Y、Z是六种短周期主族元素。元素W与元素Y位于同一主族。X、W、E三元素位于同一周期,且原子序数依次增大。D、W在周期表中的位置如...
参考答案:
(1) (2)S=C=S (3) 3NaN3 ==4N2 + Na3N ;  |
参考解析:
无

 复制链接
复制链接 新浪微博
新浪微博 分享QQ
分享QQ 微信扫一扫
微信扫一扫